Covid-19 RT-PCR Kit

Background
The new type of coronavirus 2019, namely “SARS-COV-2”, was discovered due to the 2019 viral pneumonia cases in Wuhan, and it named SARS-CoV-2 by the International Virus Classification Commission (ICTV) on February 11. Common signs of infection include respiratory symptoms, fever, cough, shortness of breath, and dyspnea. In more severe cases, infection can lead to pneumonia, respiratory failure, and even death.
According to the requirements of the “Diagnosis and Treatment Scheme for Pneumonia of New Coronavirus Infection” and “Prevention and Control Plan for Pneumonia of New Coronavirus Infection”, the 2019-Novel Coronavirus is based on real-time fluorescent RT-PCR detection of coronavirus in respiratory specimens or blood specimens as the basis for diagnosis of coronavirus.
Axbio’s newly developed 2019-Novel Coronavirus detection kit uses qRT-PCR to specifically detect the ORF1ab, N and E genes of coronaviruses. Nucleic acid detection of various samples: nasopharyngeal swabs, alveolar lavage fluid, sputum, stool, etc., with a sensitivity of 100 copies / mL
- Fast detection: Axbio one-step qRT-PCR technology uses a proprietary analysis design and amplification strategy, get detection results in 65 mins.
- Easy operation: The kit provides a one-step qRT-PCR master mix, which contains the required enzymes, primers, probes, and buffer system. Users can add a nucleic acid sample to perform the reaction.
- Strong specificity: simultaneous detection of virus ORF1ab gene, N gene and E gene, with high specificity;
- High sensitivity: High specificity and sensitivity for different samples, up to 100 copies / mL.
- Platform open: Can be applied to multiple qPCR platforms.
Product specifications:
25T, 50T
Storage conditions
-20 ° C
Sample types
Nasopharyngeal swabs, alveolar lavage fluid, sputum, feces, etc.
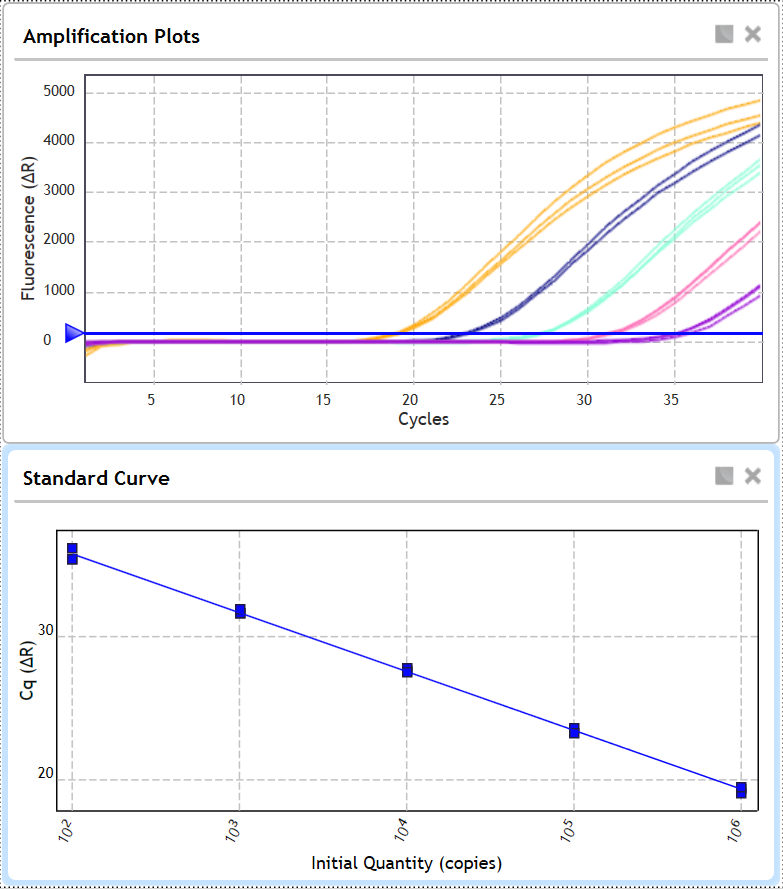
Example of Coronavirus Nucleic Acid Detection Reagent Amplification

